Plasmid DNA Development and Manufacturing
pDNA Synthesis Production and Development
Why It Matters
High-quality plasmid vectors are the essential building blocks of life-saving biologics like mRNA drugs, monoclonal antibodies and immunotherapies.
Designing diverse libraries to build plasmids from oligonucleotide fragments requires comprehensive testing. Hits must be properly screened and identified before operations are scaled.
Our Danaher Life Sciences companies support plasmid DNA (pDNA) development and manufacturing with its comprehensive platform of best-in-class science and technology companies. We recognize the necessity for solutions and aim to address these with our extensive instrument, software, and services portfolio.
The Process
One lab, three paths to faster pDNA synthesis
Resources
Method validation and references
Molecular Devices
Easy picking with the QPix 400: multiple selection modalities, wide range of microorganisms
Application Note
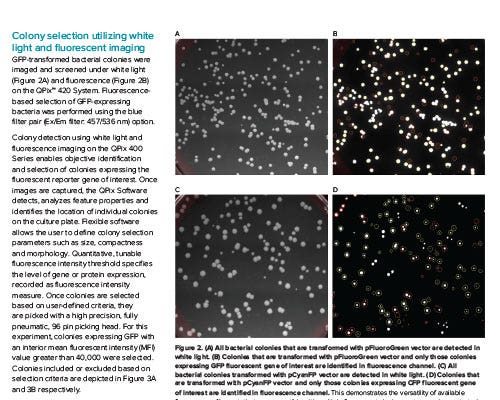
Molecular Devices
Fluorescent Bacterial Colony Selection Using QPix 400 Systems
Application Note
Beckman Coulter Life Sciences
Miniaturized Multi-Piece DNA Assembly Using the Echo 525 Liquid Handle
Application Note

Beckman Coulter Life Sciences
Nanoliter Scale DNA Assembly Utilizing the NEBuilder HiFi Cloning Kit with the Echo 525 Liquid Handler
Scientific and Technical Report

Beckman Coulter Life Sciences & Molecular Devices
Streamline your synthetic biology workflow with solutions from Beckman Coulter Life Sciences and Molecular Devices
Brochure
Product Spotlight
Echo 650 Series Acoustic Liquid Handlers
- Enables assay miniaturization in a broad range of applications
- Transfer from 384-well and 1536-well microplates
- Accurate, precise, contact-free acoustic transfer in volumes as low as 2.5 nL
- Transfer from Echo Qualified sample tubes* and microplates

FAQs
What are the challenges in plasmid DNA manufacturing?
The large-scale production of pDNA for both research and clinical purposes requires the establishment of manufacturing processes capable of yielding high quality and quantities of pDNA. Challenges unique to manufacturing and expressing plasmid-based gene therapy products emerge, particularly with complex structural DNA elements. Purification of supercoiled pDNA can be challenging without causing damage. Additionally, determining and testing the proper product (PQAs) and critical quality attributes (CQAs) can be laborious.
What are the applications of synthesized pDNA?
pDNA acts as a carrier for introducing foreign genes into host cells, facilitating the study of gene function. pDNA is also a raw material used in the manufacturing process of therapeutics like viral vectors, vaccines, and cell and gene therapies.
How is pDNA used in gene therapy?
In gene therapy, pDNA serves as a template to correct or replace defective genes. This is achieved through a process called transfection or transduction, which involves the introduction of the pDNA into the patient's cells. Once inside the cells, the pDNA is transcribed into messenger RNA, which is then translated into the correct protein. This protein then replaces or corrects the faulty protein produced by the defective gene, resulting in a functional and healthy cell.
What safety considerations are important in the handling of synthesized pDNA?
Careful management of pDNA involves selecting cell disruption methods that minimize damage to the pDNA product and prevent the shearing of host cell genomic DNA into smaller, more challenging-to-separate fragments. Membrane filtration, a widely used separation method for mass-producing pDNA, requires strict adherence to safety protocols for handling filtration equipment and materials. Safety considerations extend beyond production and handling to encompass the use of physical techniques for gene delivery, minimizing immune responses and designing plasmid expression systems with attributes like tissue specificity, self-replication and integration to support enduring gene expression.