Proteins are intricate biological molecules composed of one or more elongated and folded chains known as polypeptides. These polypeptides are constructed based on the DNA sequence found in the gene responsible for encoding the protein.
Proteins play vital roles in various cellular processes, such as providing structural stability, acting as catalysts for biochemical reactions, functioning as hormones and enzymes, contributing to cellular growth and development, initiating programmed cell death, facilitating signaling, transport, and cell adhesion.
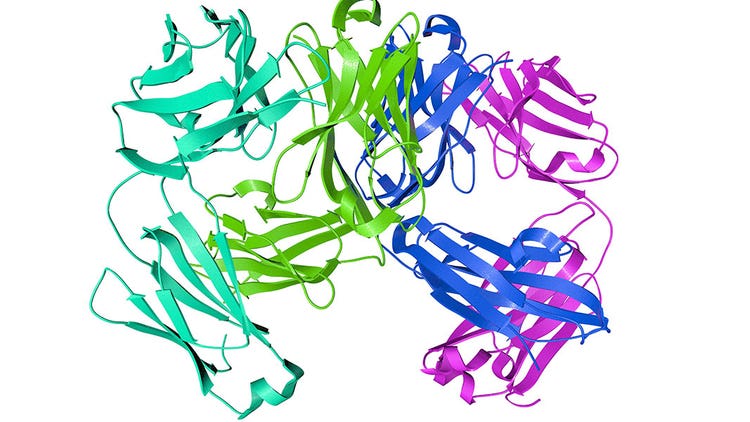
Structural Biology of Proteins
- Amino Acids and Peptide Bonds: Amino acids are the building blocks of proteins and are connected through peptide bonds, forming a linear chain called a polypeptide. Peptide bonds are created through a chemical reaction where the carboxyl group of one amino acid reacts with the amino group of the adjacent amino acid, leading to their linkage. Different structures of proteins form through the intricate folding and arrangement of the polypeptide chains, driven by various chemical interactions and forces.
- Primary Structure: Proteins are constructed through a sequential arrangement of amino acid residues linked by peptide bonds. The linear order of these residues forms the backbone of the protein structure.
- Secondary Structure: Proteins exhibit various shapes including alpha helixes, beta-pleated sheets and beta-turns. Hydrogen bonds stabilize these secondary structures.
- Tertiary Structure: The tertiary structure of proteins emerges when the polypeptide chain interacts with the surrounding aqueous environment, forming shortly after synthesis and exiting the ribosomal subunit. It involves the sequestration of hydrophobic residues and exposure of hydrophilic ones to achieve thermodynamic stability.
- Quaternary Structure: The quaternary structure of proteins refers to the arrangement and interaction of multiple protein subunits to form a functional, complex protein assembly.
See how Danaher Life Sciences can help
Protein Synthesis
- Transcription: Transcription comprises initiation, elongation, and termination stages. Initiation involves binding RNA polymerases to a specific region called the promoter on DNA, signaling the start of transcription. Elongation entails RNA polymerases moving along the DNA template, unwinding the DNA helix, and assisting in mRNA synthesis by adding complementary nucleotides. Finally, termination occurs when RNA polymerases encounter a termination sequence, leading to their dissociation from DNA and the release of the newly formed RNA molecule.
- Translation: Translation is the ribosome-mediated synthesis of proteins using mRNA and tRNA. It involves initiation with small ribosomal subunit binding, start codon recognition by initiator tRNA, and large ribosomal subunit joining. Elongation occurs as the ribosome moves along mRNA, reads codons, and adds amino acids via tRNA to form a polypeptide chain. Termination happens at the stop codon, releasing the polypeptide and disassembling the ribosome. The synthesized protein then folds into its functional structure.
- Post-Translational Modifications: These are chemical modifications that occur after protein synthesis, altering the characteristics of a protein. These modifications involve proteolytic cleavage and adding various modifying groups, such as acetyl, phosphoryl, glycosyl, and methyl groups, to one or more amino acids within the protein.
Protein Folding and Misfolding
Protein folding is the complex process in which a protein undergoes a series of steps to attain its biologically active three-dimensional structure. This is achieved through formation of stable secondary and tertiary structures, and stabilization of various interactions crucial for its proper function. Protein folding requires assistance from chaperone proteins and other cellular factors.
Chaperones are a group of proteins that play a crucial role in assisting protein folding by promoting correct folding pathways, preventing misfolding and aggregation, and facilitating the attainment of proper protein structure and function.
However, protein folding is prone to error which can have biological consequences. Alzheimer's and Parkinson's are characterized by the accumulation of abnormally folded proteins in the body, leading to neurodegeneration and functional impairment.
Functions of Proteins in Biology
Enzymes are proteins that catalyze chemical reactions in cells, speeding up the rate of specific biochemical reactions. Structural proteins provide support and shape to cells and tissues, forming the framework for various biological structures. Another type of protein called transport protein facilitates the movement of molecules and ions across cell membranes, enabling essential processes such as nutrient uptake and waste removal. Playing a crucial role in cell communication and coordination, signaling proteins help transmit signals within and between cells. Furthermore, the biological role of regulatory proteins is to control gene expression and other cellular processes, regulating the timing and intensity of various biological activities.
Protein-Protein Interactions
- Protein Complexes: Protein complexes are formed when multiple proteins bind together, functioning as a cohesive unit to carry out specific biological processes.
- Protein Binding and Recognition: Proteins possess the ability to bind to specific molecules or other proteins through precise recognition mechanisms, allowing for the formation of functional complexes and the initiation of various cellular activities.
- Protein Interaction Networks: Protein interaction networks represent the intricate web of physical and functional interactions among proteins within a cell or organism, providing insights into the organization and coordination of biological processes.
Techniques in Protein Biology
- Protein Purification: Protein purification involves isolating a specific protein or a group of proteins from a complex mixture, allowing for a detailed study of its structure, function, and interactions.
- Protein Analysis Methods: SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and Western blotting are commonly used techniques to separate and detect proteins. These techniques can inform on a protein’s size, quantity and identity (e.g., Western blot using antibody recognition).
- Protein Characterization Techniques: Mass spectrometry is a powerful technique used to determine the mass, structure and composition of proteins. Insights gleaned are post-translational modifications, protein folding and identification of protein-protein interactions.
Protein crystallography is a microscopy technique allowing for structural characterization at the atomic level.
Protein Engineering and Applications
Rational protein design modifies proteins based on structural and functional knowledge to enhance stability, activity, or desired properties.
Directed evolution mimics natural selection to optimize proteins for specific applications by generating diverse mutations and selecting superior variants based on desired properties.
Proteins have diverse applications in biotechnology, including industrial enzymes for food processing and biofuel production, and therapeutic proteins like antibodies, growth factors, and enzymes are useful in clinical treatment.
Future Directions in Protein Biology
Proteomics will continue contributing to the understanding of biological structures, functions and networks of proteins within cells and organisms. Proteomics will be fueled by advancements in computational modeling to help map previously unknown protein-protein interactions, roles in cellular signaling networks, and identify potential disease specific protein biomarkers. In conjunction with protein engineering, new biologics for therapeutic use will also continue to be a focus.
See how Danaher Life Sciences can help
Protein Biology