We See a Way to
reduce experiment execution by 7000 hours
IDBS | Polar
Don’t let poor data management, lack of data access and missing context cost you thousands of hours in redundant work.

IDBS Polar: Streamline planning and execution by integrating key data
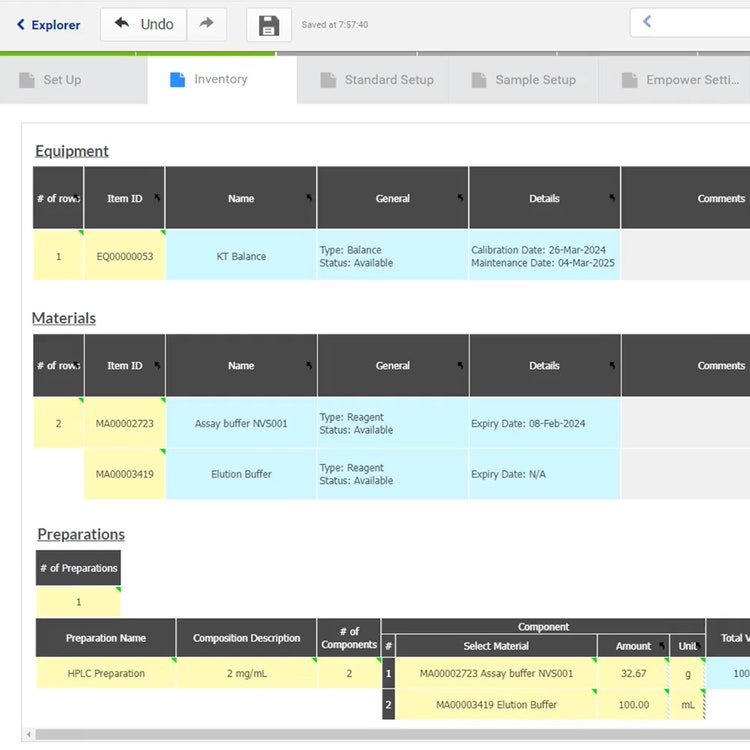
Streamline experiment execution with IDBS Polar
IDBS Polar is a unified lab informatics platform that integrates ELN, LIMS and LES capabilities to securely and compliantly control experiment execution, manage material and sample lifecycle and genealogy while connecting seamlessly to experiments. Polar ensures process consistency and reproducibility with flexible, scalable low-code/no-code solutions for structured data capture and procedure management capabilities.
Leverage high-quality data to accelerate scientific outcomes
Fully compliant with FDA 21 CFR Part 11 and EudraLex Volume 4 Annex 11, IDBS Polar offers extensive data capture, instrument integration and reporting capabilities across the drug development workflow—making it the informatics backbone for data- and AI-driven science.

Scalable and reproducible workflows to accelerate time to market
Based on over 35 years of industry expertise and design best practices, IDBS has developed a library of Accelerators for Analytical Development teams to capture, analyze and report on data. These Accelerators enable the capture of structured, catalog-mapped data at the point of experiment execution for quantitative, qualitative and operational assays and provide reporting and statistical analyses for process and product insight and faster data-driven decision making.
Speak to one of our leading life sciences experts
TTAE
TTAE
546006278
TTAE
add
Talk to an Expert
https://lifesciences.danaher.com/
https://lifesciences.danaher.com/us/en/solutions/mabs/cell-line-development.html
E-WorkBook customer data on file at IDBS. IDBS Polar delivers the same or higher gains as IDBS E-WorkBook in a like-for-like implementation, with added value from advanced analytics, AI/ML and reporting.