A Powerful Tool for Silencing Genes and Treating Disease
Small (or short) interfering RNA (siRNA) is a double-stranded RNA molecule that affects gene expression by silencing genes. siRNA plays a major role in post-transcriptional gene silencing (PTGS) by binding to a homologous messenger RNA (mRNA) and triggering its destruction, thereby preventing it from being translated into protein. This mechanism is referred to as RNA interference (RNAi) and has therapeutic application for slowing or preventing disease development and progression. In addition to gene silencing, siRNAs are known to play a role in chromosome organization and antiviral defense.
RNA interference (RNAi): The Natural Process of Gene Silencing
RNAi was discovered in the 1990s and is a natural process that occurs in cells to regulate gene expression. siRNAs were identified soon after as the molecule responsible for RNAi. They are naturally occurring double-stranded RNA molecules typically 20-25 nucleotides in length but can also be synthetically manufactured using modified nucleotides.
siRNA Processing and RNA-Induced Silencing Complex (RISC)
siRNAs can be introduced to cells via a viral vector where it is processed and loaded into the RNA-induced silencing complex (RISC) and activates the RNAi process. The RISC uses the siRNA antisense strand to identify the complementary mRNA target and then cleaves it, leading to the degradation of the mRNA and a decrease in the expression of the target gene.
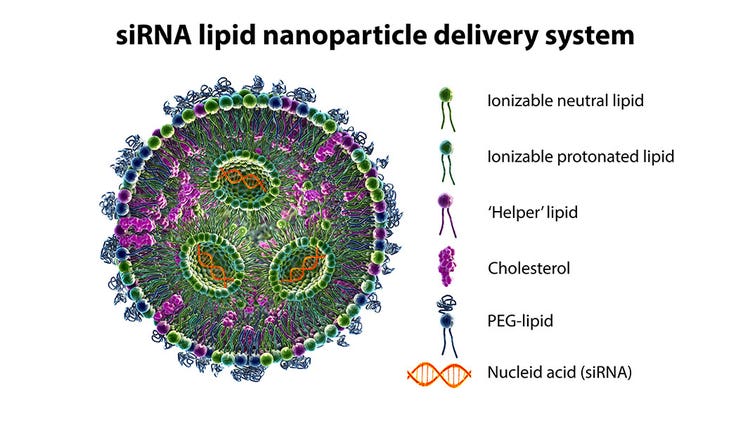
Advancements in siRNA Therapeutics: Enhancing Stability, Delivery, and Efficacy
Significant progress has been made in the development of siRNA-based therapeutics over the past decade. Improvements include increased half-lives of siRNAs with modified nucleotide incorporation, better cell targeting and delivery methods, and decreased immunogenicity and off-target effects.
Enhancing siRNA Pharmacological Properties
Small molecules can be conjugated to siRNAs to improve its pharmacological properties. Below are a few examples.
- GalNAc: GalNAc (N-acetylgalactosamine) has been shown to enhance the cellular uptake and intracellular localization of siRNA, leading to increased efficacy in RNA interference-based therapies. The targeted delivery of GalNAc-siRNA conjugates to specific cells or tissues can minimize off-target effects and reduce the risk of toxicity.
- PEGylation: Polyethylene glycol (PEG) is the most widely used small molecule for siRNA conjugation. PEGylation can increase the stability and circulation time of siRNA in the bloodstream, thereby reducing the required frequency of administration and increasing the therapeutic efficacy of siRNA.
- Cholesterol: Cholesterol can be conjugated to siRNA to form lipoplexes, improving the stability and uptake of siRNA into cells.
- Antibodies: Antibodies specific to a target cell type can be conjugated to siRNA to increase the specificity and efficacy of RNAi-based therapies.
- Peptides: Peptides, such as cell-penetrating peptides (CPPs) and tumor-homing peptides, can be conjugated to siRNA to enhance the cellular uptake and delivery of siRNA to specific tissues.
- Lipid nanoparticles: Lipid nanoparticles can be used to encapsulate siRNA to improve its stability, increase its circulation time and enhance its delivery to target cells.
These small molecule conjugates can significantly enhance the pharmacological properties of siRNA, making it a more promising therapeutic strategy for various diseases.
See how Danaher Life Sciences can help
siRNA Applications
Applications of siRNA in Research and Therapeutics
siRNA has wide-ranging applications in areas such as functional genomics, disease modeling, drug discovery and therapeutics. Listed below are a few examples of how they are applied.
- Gene knockdown experiment s: Introduction of siRNA molecules that target specific genes to reduce their expression and study the resulting phenotypic changes in cells or organisms. This technique is used to study the function of genes in many biological processes, such as cellular signaling pathways, disease development and cell differentiation.
- Functional genomics: Compares the phenotype of cells treated with different siRNAs to determine which genes are involved in specific cellular processes and pathways.
This approach can help identify new drug targets and the molecular mechanisms underlying complex biological processes.
- Disease modeling: Reduction of specific gene expression in cells or animal models allows the study of pathogenesis and the testing of new therapeutic strategies. Disease-specific screens with a siRNA library can be used to determine the identity of the genes involved in the development of a particular disease.
- Toxicity testing: siRNA can be used to test the toxicity of small molecule compounds and biologics in cells. By reducing the expression of genes involved in cellular stress responses, researchers can determine the impact of a potential drug on cellular health and viability.
Potential Therapeutic Applications of siRNA: Targeting Disease at its Root
The ability of siRNA to specifically target and reduce the aberrant expression of disease-causing genes makes it an attractive therapeutic strategy.
- Cancer: siRNA can be used to silence oncogenes that drive the growth and spread of cancer cells. By reducing the expression of these genes, siRNA can inhibit the growth and progression of cancer cells.
- Viral infections: siRNA can be used to target specific viral genes and reduce their expression during viral infections.
- Genetic disorders: siRNA can be used to treat genetic disorders, including inherited diseases, by targeting specific disease-causing genes and reduce their expression.
In addition to these diseases, siRNA is being explored as a potential therapeutic approach for other diseases, including neurodegenerative, cardiovascular and autoimmune diseases.
FDA Approved siRNA Drugs for Clinical Use: Paving the Way for the Future
Patients are currently benefiting from siRNA-based therapeutics to treat various rare genetic diseases and indicate the potential for a wide range of diseases, including cancer, viral infections and genetic disorders.
Examples of approved siRNA therapeutics:
- Patisiran is a siRNA-based drug that targets the transthyretin (TTR) gene, which is associated with hereditary transthyretin-mediated amyloidosis (hATTR), a rare genetic disease. Patisiran was approved by the US Food and Drug Administration (FDA) in 2018 and is the first approved siRNA therapeutic.
- Inclisiran is a siRNA-based drug that targets proprotein convertase subtilisin/kexin type 9 (PCSK9), a protein involved in regulating cholesterol levels in the blood. Inclisiran was approved by the FDA in 2021 and is used to lower cholesterol levels in patients with cardiovascular disease.
- Lumasiran is a siRNA-based drug that targets the hydroxyacid oxidase 1 (HAO1) gene, which is associated with primary hyperoxaluria type 1 (PH1), a rare genetic disease. Lumasiran was approved by the FDA in 2020 and is the first siRNA therapeutic approved for treating PH1.
Challenges and Future Applications of siRNA
siRNA therapy, has shown great potential in preclinical studies and some clinical trials. However, challenges still remain in its clinical translation.
- One major challenge is the efficient delivery of siRNA molecules to target cells, tissues and organs, which requires overcoming various biological barriers.
- Additionally, off-target effects and immune responses can limit the therapeutic efficacy and safety of siRNA.
Nonetheless, significant progress is being made in developing more efficient delivery methods, optimizing siRNA sequences and reducing off-target effects. Furthermore, the FDA has recently approved several siRNA-based therapies, paving the way for future developments in this field.
See how Danaher Life Sciences can help
Small Interfering RNA (siRNA)